Listen to the audio blog
Medical disclaimer: This article is for education only and is not medical advice. Always consult your clinician for personal guidance.
The Technological Renaissance in Preventive Cardiology – Artificial Intelligence, Quantitative Coronary Tomography
Introduction
Cardiovascular medicine is undergoing a substantive paradigm shift, moving from a predominantly reactive, symptom-driven model toward a preventive framework grounded in early detection and individualized risk assessment. At the center of this transition is the integration of artificial intelligence (AI) with coronary computed tomography angiography (CCTA), particularly through the development of atherosclerosis imaging–quantitative computed tomography (AI-QCT). This approach addresses a long-standing limitation in cardiology: the inability of traditional diagnostic tools to reliably detect non-obstructive, lipid-rich coronary plaque, despite such lesions being responsible for the majority of acute coronary syndromes and sudden cardiac death events (1).
The clinical relevance of this limitation is substantial. Epidemiologic and pathologic studies consistently demonstrate that most myocardial infarctions arise from plaques that were not flow-limiting prior to rupture (2). As cardiovascular disease remains the leading cause of mortality worldwide, with annual deaths projected to exceed 20 million by 2030, improved methods for identifying high-risk coronary atherosclerosis before clinical events occur are increasingly necessary (3).
Evolution of Cardiac Risk Stratification and the Biology of Atherosclerosis
Traditional cardiovascular risk stratification relies on indirect markers such as serum lipid levels, blood pressure, glycemic control, smoking status, and demographic variables incorporated into population-based risk calculators. While these models have proven useful at a population level, they often lack precision when applied to individual patients, particularly those with apparently “normal” risk factor profiles who nonetheless harbor significant subclinical disease (4).
Atherosclerosis is a chronic inflammatory disease of the arterial wall rather than a disorder defined solely by luminal obstruction. Plaque develops within the intima and consists of varying proportions of lipid, fibrous tissue, inflammatory cells, and calcium. Conventional diagnostic tests—including exercise stress testing, electrocardiography, and invasive coronary angiography—primarily detect disease once it produces hemodynamically significant stenosis, typically defined as luminal narrowing of 70% or greater (5).
Insight 1: Autopsy and angiographic studies demonstrate that approximately 70–75% of myocardial infarctions occur in vessels with less than 50% stenosis prior to the index event, indicating that most patients who experience acute coronary syndromes would not have been identified as high risk by standard stress testing alone (2).
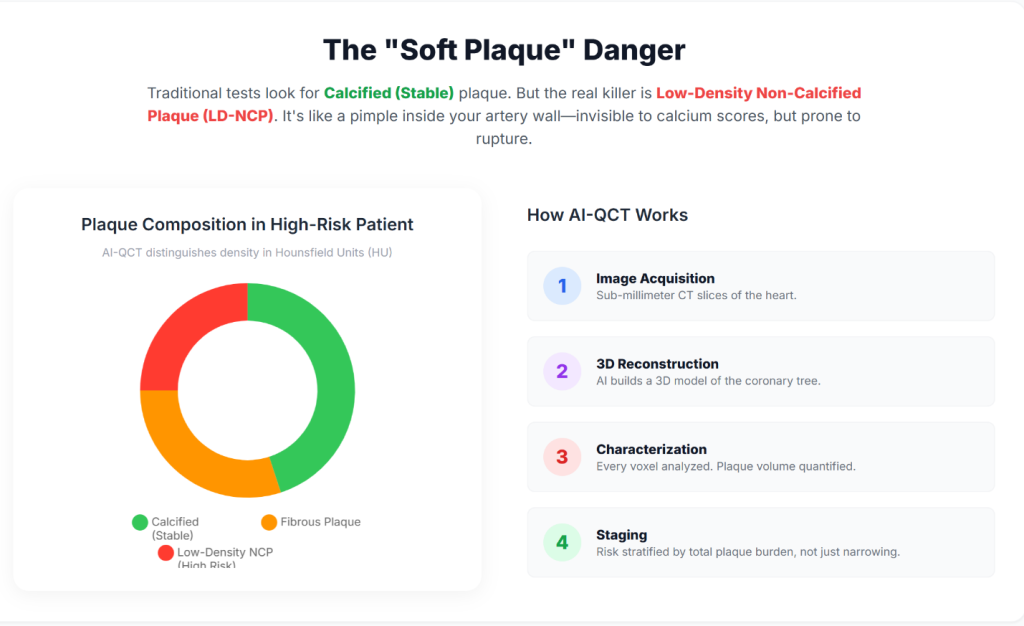
Insight 2: Coronary artery calcium scoring (CACS) was developed to improve detection of subclinical disease by identifying calcified plaque. However, CACS does not detect non-calcified plaque, and a calcium score of zero does not exclude the presence of lipid-rich, rupture-prone lesions (6).
Insight 3: AI-QCT enables non-invasive quantification of total coronary plaque burden, including low-density non-calcified plaque, which has been shown to carry a higher association with future cardiovascular events than stenosis severity alone (7).
| Plaque Characteristic | Visibility (CACS) | Visibility (Standard CCTA) | Visibility (AI-QCT) | Clinical Risk Profile |
| Calcified plaque | High | High | High | Generally stable; marker of chronic disease |
| Non-calcified plaque | None | Qualitative / limited | Quantitative / high | Elevated rupture risk |
| Low-density plaque | None | Variable | High | Strong association with ACS |
| Total plaque volume | None | Indirect estimate | Precise (mm³) | Strong predictor of future events |
Technical Architecture of AI-Enabled Coronary Analysis
AI-QCT platforms perform voxel-level analysis of the coronary tree rather than relying on visual interpretation alone. These systems employ deep-learning architectures—including convolutional neural networks (CNNs), three-dimensional U-Net segmentation models, and VGG-derived classifiers—trained on large, curated datasets that link imaging features with invasive validation and long-term clinical outcomes (8).
Insight 4: AI-QCT begins with acquisition of high-resolution CCTA. Contemporary multi-detector CT systems, including 64- and 640-slice scanners, provide the temporal and spatial resolution necessary to minimize motion artifact and enable accurate segmentation of coronary anatomy (5).
Insight 5: Automated algorithms identify the coronary lumen and vessel wall and classify plaque components according to Hounsfield unit attenuation values, allowing differentiation of lipid-rich, fibrous, and calcified tissue (9).
Insight 6: AI-derived ischemia indices integrate plaque morphology, lesion length, vessel remodeling, and luminal geometry to estimate flow limitation, providing a non-invasive correlate of invasive fractional flow reserve (FFR) (10).
Clinical Validation and Peer-Reviewed Evidence
AI-QCT has been evaluated in multiple prospective trials, registries, and comparative studies published in leading cardiovascular and imaging journals, including Journal of the American College of Cardiology, Circulation: Cardiovascular Imaging, and European Heart Journal – Cardiovascular Imaging (11).
Landmark Trials and Registries
Insight 7: The CREDENCE trial demonstrated close agreement between AI-assisted CCTA analysis and invasive quantitative coronary angiography and FFR, supporting the diagnostic accuracy of AI-QCT for identifying hemodynamically significant disease (12).
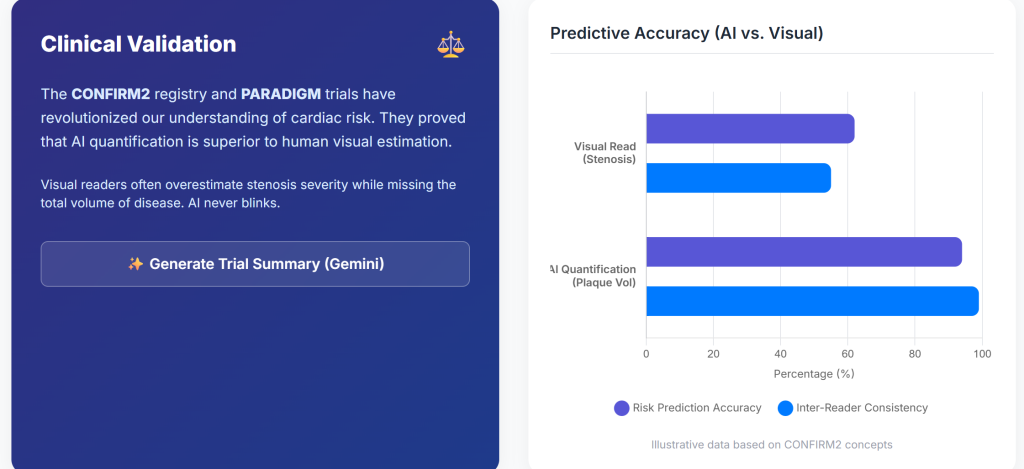
Insight 8: The CONFIRM2 registry, encompassing tens of thousands of patients across multiple countries, showed that AI-quantified plaque features identify a wide gradient of cardiovascular risk among patients with non-obstructive coronary artery disease (7).
Insight 9: The CERTAIN study demonstrated that AI-QCT significantly improved diagnostic certainty compared with standard interpretation, resulting in more appropriate medical therapy and fewer unnecessary downstream tests (1).
| Study | N | Key Finding | P-value | Clinical Implication |
| CONFIRM2 | 6,550 | AUC for MACE improved from 0.62 to 0.75 | <0.001 | Improved prognostic accuracy |
| CREDENCE | 513 | High concordance with invasive FFR | <0.01 | Viable non-invasive ischemia assessment |
| PACIFIC-1 | 208 | AI exceeded expert readers | <0.05 | Reduced inter-observer variability |
| PROMISE (subset) | 4,347 | 41% stenoses reclassified | — | Reduced false positives |
Preventive Cardiology Implementation and Longitudinal Disease Monitoring
Integration of AI-QCT into preventive cardiology workflows shifts the clinical focus from episodic evaluation toward longitudinal monitoring of atherosclerotic disease.
Insight 10: Quantification of total plaque burden enables objective assessment of disease progression or regression over time, facilitating a treat-to-target approach guided by serial imaging (7).
Insight 11: AI-based comparison tools permit evaluation of changes in plaque composition, including stabilization or regression of lipid-rich plaque in response to medical therapy (13).
Hardware Advances: Photon-Counting Computed Tomography
The performance of AI-QCT is dependent on the quality of input imaging data, which continues to improve with advances in CT hardware.
Insight 12: Photon-counting CT (PCCT) systems directly convert X-ray photons into electrical signals, reducing electronic noise and calcium blooming artifacts compared with conventional energy-integrating detectors (14).
Insight 13: PCCT provides higher spatial resolution with lower radiation and contrast dose, expanding eligibility for coronary CT imaging to patients with high heart rates, elevated body mass index, or extensive coronary calcification (15).
Economic and Health System Implications
The adoption of AI-enabled cardiac imaging aligns with broader healthcare trends toward value-based care and prevention.
Insight 14: Market analyses project rapid growth of AI applications in cardiology, driven by improved diagnostic efficiency and potential reductions in downstream costs associated with acute coronary events (16).
Insight 15: Early identification of high-risk plaque phenotypes may reduce myocardial infarction rates and associated healthcare expenditures by enabling earlier and more targeted intervention (17).
Conclusions and Future Directions
Preventive cardiology is increasingly transitioning toward a biologically informed model that emphasizes plaque burden and composition rather than stenosis severity alone. AI-QCT provides a reproducible, quantitative framework for assessing coronary atherosclerosis before clinical events occur.
Insight 16: Future developments are likely to include integration of agentic AI systems, longitudinal digital heart models, and multimodal data streams incorporating genomic and metabolic information (18).
Insight 17: As AI assumes a greater role in image interpretation and quantification, clinicians may increasingly focus on higher-level clinical decision-making, risk communication, and individualized therapy optimization (19).
References
- Nurmohamed NS, Cole JH, Budoff MJ, et al. Impact of atherosclerosis imaging–quantitative computed tomography on diagnostic certainty, downstream testing, coronary revascularization, and medical therapy: the CERTAIN study. Eur Heart J Cardiovasc Imaging. 2024;25(6):857-866.
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657-671.
- World Health Organization. Cardiovascular diseases (CVDs). 2023.
- Lloyd-Jones DM, et al. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2019;140:596-608.
- Douglas PS, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
- Budoff MJ, et al. Coronary artery calcium scoring and cardiovascular risk assessment. J Am Coll Cardiol. 2018;72:434-447.
- Feuchtner G, et al. AI-quantitative CT coronary plaque features associate with higher relative risk: CONFIRM2 Registry. Circ Cardiovasc Imaging. 2025.
- Dey D, et al. Machine learning in cardiovascular CT. JACC Cardiovasc Imaging. 2020;13:258-275.
- Lin A, et al. Quantitative coronary plaque assessment by CT. Eur Heart J. 2021;42:490-499.
- Nørgaard BL, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary CT angiography. J Am Coll Cardiol. 2014;63:1145-1155.
- Cleerly Health. Clinical publications. 2025.
- Choi AD, et al. AI evaluation of coronary stenosis: CREDENCE substudy. JACC Cardiovasc Imaging. 2022.
- Williams MC, et al. Plaque modification assessed by coronary CT. Heart. 2022;108:1033-1040.
- Willemink MJ, et al. Photon-counting CT: technical principles and clinical applications. Radiology. 2021;298:567-580.
- Si-Mohamed S, et al. Photon-counting CT in cardiac imaging. Eur Radiol. 2022;32:537-548.
- Grand View Research. Artificial intelligence in cardiology market report. 2024.
- Neale T. Evidence around AI in cardiology grows. TCTMD. 2025.
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56.
- Shah SJ, et al. Precision medicine in cardiology.
AI Use Notice: Portions of this article were drafted with the assistance of AI tools. The author reviewed and edited the content, verified sources where applicable, and is responsible for the final version. This content is for educational purposes and is not medical advice.